Researchers
Prof. Santiago Alvarez, Dr. Gabriel Aullón
Our research on stereochemistry, bonding and reactivity deals mostly with transition metals, dealing with the geometric factors that affect multiple metal-metal bond strength, or delocalized bonding in M2X2 diamonds, M3X2 and M2X3cages. The study of the relationship between stereochemistry and spin state has led us to formulate simple rules to predict the geometries of four and six-coordinated transition metals.
We are also concerned about intermolecular bonding, from d10···d10metallophilic interactions to homopolar dihydrogen bonding in dimers of simple alkanes and polyhedranes. Using continuous shape measures methodology, we have also contributed to precise stereochemical description of metal complexes with ill-defined coordination numbers due to the weak coordination of one or more ligands. Also comprehensive structural analysis of non bonded intermolecular distances in crystal structures has allowed us fo propose a cartography of the Van der Waals territory and a new set of Van der Waals radii. Related studies have also resulted in a proposal of an coordination ability index for a wide number of anions and solvents.
Main Results of Our Research in this Field
- Reviews
- Van der Waals interactions
- Metallophilic interactions
- Delocalized bonding in metal-ligand rings and cages
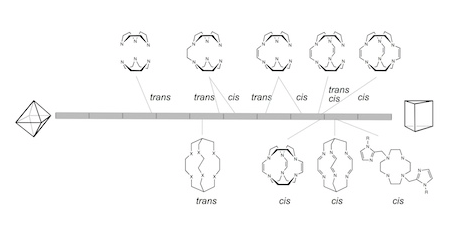
- Stereo-spin isomerism of coordination compounds
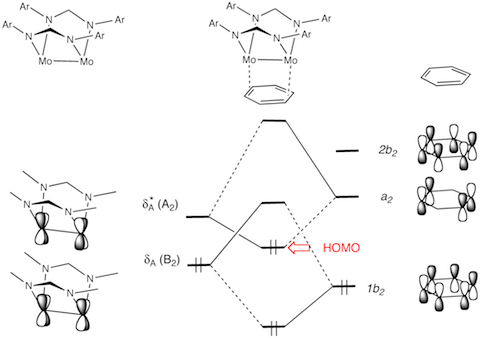
- Metal-metal bonding
Data Available for Download:
- Table of covalent radii
- Table of coordination ability indexes of anions and solvents
Relevant Publications
| S. Alvarez, E. Ruiz. «Self-Assembly of Coordination Compounds: Design Principles», in Supramolecular Chemistry, From Molecules to Nanomaterials, J. W. Steed, P. A. Gale, eds., John Wiley & Sons, Chichester, UK, 2012, 5, 1993-2044. ISBN 978-0-470-74640-0 |
| E. Ruiz, J. Cirera and S. Alvarez, «Spin density distribution in transition metal complexes», Coord. Chem. Rev. 2005, 249, 2649-2660. |
| S. Alvarez, «Bonding and stereochemistry of three-coordinated transition metal compounds», Coord. Chem. Rev. 1999, 193-5, 13-41. |
All Publications of Stereochemistry, Bonding and Reactivity of Transition Metal Compounds.